News Center
2022-11-03
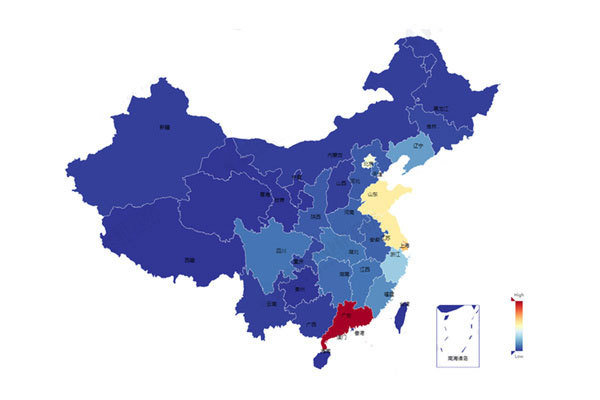
The national medical device inspection officially started
Xu Jinghe, a member of the Party Leadership Group and Deputy Director of the State Food and Drug Administration, stressed that we should pay close attention to the quality supervision of medical devices and make every effort to ensure the quality and safety of medical devices for epidemic prevention and control.
Manufacturers of medical devices for epidemic prevention and control should fully implement the main responsibility of enterprises, strengthen the sense of responsibility, and further enhance the consciousness of responsibility implementation; Strengthen the system management to ensure the continuous compliance of the quality management system; Risk disposal shall be strengthened, and the quality risks found shall be disposed at the first time.
All provincial drug regulatory departments should, in accordance with the "four strictest" requirements, earnestly implement their local regulatory responsibilities, strengthen law enforcement inspection, product sampling inspection, adverse event monitoring and risk troubleshooting, and strengthen interaction with relevant departments to form a joint regulatory force.
Related News